Atoms of a low-pressure gas can occur in certain energy states. The lowest energy state E1iscalled groundstate. The atom may accept certain amounts of energy. The atom will put in an excited state. The orbit of the electron becomes a larger radius
Higher energy levels are the 1e, 2 e, ….. exited states. E2 ,E3 enz
The energy levels of the H-atom are :
E∞ = 0.0 eV ionized state
————–
E3 = -1.5 eV 2e exited state
E2 = -3.4 eV 1e exited state
E1 = – 13.6 eV groundstate
Remark: between the 2e excited state and the ionized state are many more energy levels.
Whenever a atom falls back to a lower energy level the surplus energy is releases as a photon.
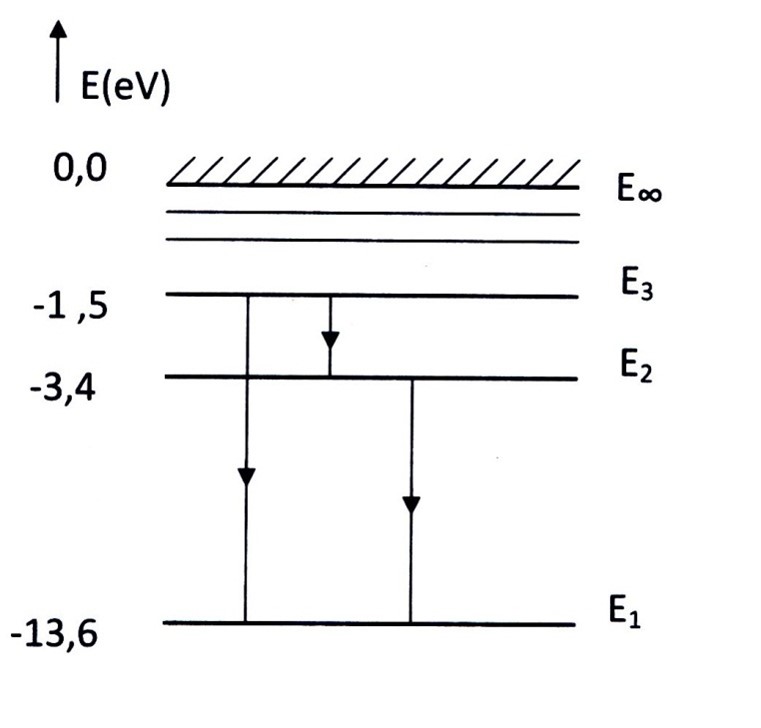
Example
A H-atom is in the 2e excited state.
The atom falls back to the ground state.
There are 2 posibilities:
- Direct from E3 to E1 (ΔE =E3– E1=-1.5- -13.6 = 12.1 eV)
- From E3 to E2 (ΔE = -1.5- – 3.4 = 1.9 eV ) . Then follows E2 to E1
(- 3.4 – – 13.6 = 10.2 eV)
Calculate the wavelength corresponding the transition of 1.9 eV (E3→E2)
ΔE = 1.9 eV = 1.9 x 1.6 x 10-19 = 3.04 x 10-19 J
ΔE=h.f 3.04 x 10-19 = 6.63 x 10-34 . f f=3.04 x 10-19 / 6.63 x 10-34 = 4.59 x 1014 Hz
c = f.λ 3.00 x 108 = 4.59 x 1014 .λ λ = 3.00 x 108 /4.59 x 1014 = 6.54 x 10-7 m = 654 nm
This is the red line of the H-spectrum.
Every energy transition is corresponding with a certain frequency (or wavelength)